

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM28673 5-chloro-2-({3-[(5-hydroxy-1,2,3,4-tetrahydroisoquinoline-2-)sulfonyl]benzene}amido)benzoic acid::Anthranilic acid deriv., 12
SMILES: OC(=O)c1cc(Cl)ccc1NC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(O)cccc2C1
InChI Key: InChIKey=GEBUDDVQSUDCAD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28673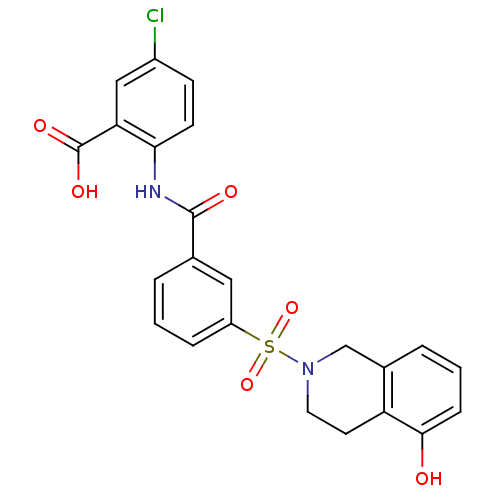 (5-chloro-2-({3-[(5-hydroxy-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | 2.00E+3 | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM28673 (5-chloro-2-({3-[(5-hydroxy-1,2,3,4-tetrahydroisoqu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM28673 (5-chloro-2-({3-[(5-hydroxy-1,2,3,4-tetrahydroisoqu...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GSK | Assay Description Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... | Bioorg Med Chem Lett 18: 5018-22 (2008) Article DOI: 10.1016/j.bmcl.2008.08.011 BindingDB Entry DOI: 10.7270/Q2WD3XWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||