

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM288492 (1R,2R,5R)-5-[8- amino-1-(4-{[4- (trifluoromethyl) pyridin-2- yl]carbamoyl}phenyl) imidazo[1,5-a]pyrazin- 3-yl]-2-(1- methylethyl) cyclohexane- carboxylic acid::US10087188, Example 99
SMILES: CC(C)[C@H]1CC[C@H](C[C@H]1C(O)=O)c1nc(-c2ccc(cc2)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12
InChI Key: InChIKey=PAQMIHDIOFLBAZ-HMXCVIKNSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288492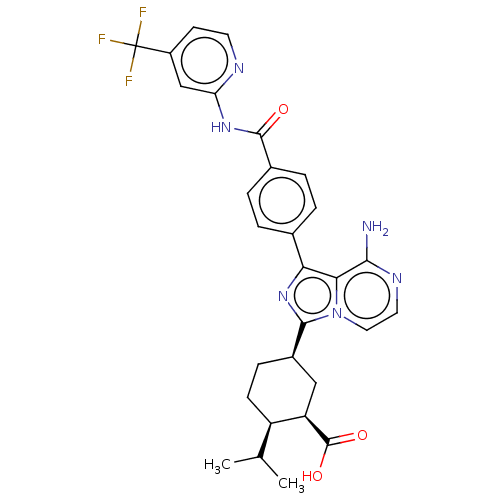 ((1R,2R,5R)-5-[8- amino-1-(4-{[4- (trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US10087188 (2018) BindingDB Entry DOI: 10.7270/Q2GF0WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288492 ((1R,2R,5R)-5-[8- amino-1-(4-{[4- (trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co Inc Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC | Bioorg Med Chem Lett 27: 1471-1477 (2017) BindingDB Entry DOI: 10.7270/Q20867K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288492 ((1R,2R,5R)-5-[8- amino-1-(4-{[4- (trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co Inc Curated by ChEMBL | Assay Description Inhibition of 6-His-tagged recombinant full length BTK (unknown origin) expressed in baculovirus-transfected Sf9 cells using Biotin-EQEDEPEGDYFEWLE-N... | Bioorg Med Chem Lett 27: 1471-1477 (2017) BindingDB Entry DOI: 10.7270/Q20867K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||