

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM288560 4-[8-amino-1-(4-{[4- (trifluoromethyl) pyridin-2- yl]carbamoyl}phenyl) imidazo[1,5-a]pyrazin- 3- yl]bicyclo[2.2.2] octane-1-carboxylic acid::US10087188, Example 168
SMILES: Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)C12CCC(CC1)(CC2)C(O)=O
InChI Key: InChIKey=XTLSAZFITBCMQC-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288560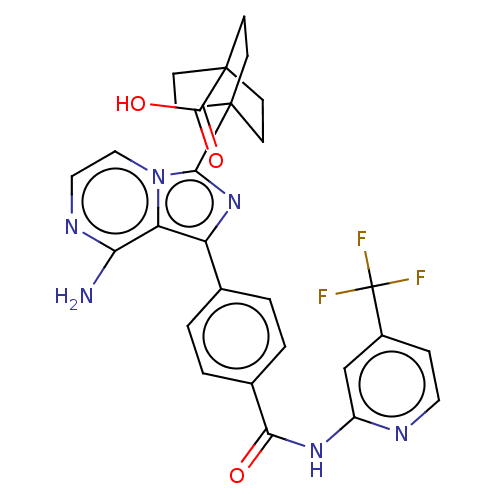 (4-[8-amino-1-(4-{[4- (trifluoromethyl) pyridin-2- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US10087188 (2018) BindingDB Entry DOI: 10.7270/Q2GF0WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288560 (4-[8-amino-1-(4-{[4- (trifluoromethyl) pyridin-2- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co Inc Curated by ChEMBL | Assay Description Inhibition of 6-His-tagged recombinant full length BTK (unknown origin) expressed in baculovirus-transfected Sf9 cells using Biotin-EQEDEPEGDYFEWLE-N... | Bioorg Med Chem Lett 27: 1471-1477 (2017) BindingDB Entry DOI: 10.7270/Q20867K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM288560 (4-[8-amino-1-(4-{[4- (trifluoromethyl) pyridin-2- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co Inc Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC | Bioorg Med Chem Lett 27: 1471-1477 (2017) BindingDB Entry DOI: 10.7270/Q20867K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||