

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N[C@H]1C[C@@H]1O
InChI Key: InChIKey=LZHCSJBKBARIFQ-NILUWUTCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29088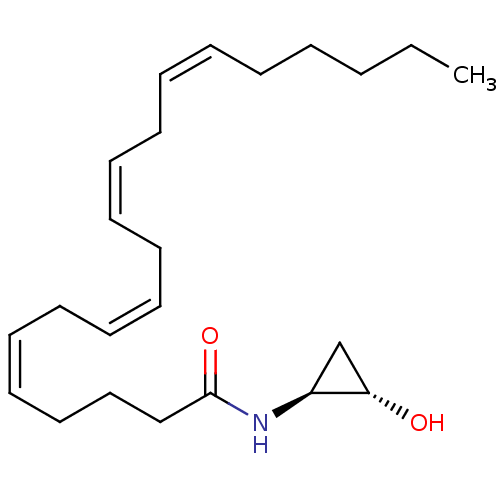 (cyclopropanolamide, 11a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29088 (cyclopropanolamide, 11a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM29088 (cyclopropanolamide, 11a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description The effect of the substances on Ca2+ influx was determined by using HEK-293 cells stably overexpressing recombinant human TRPV1 cDNA. The cells were ... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||