

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM29234 CHEMBL481815::US9090589, 6::aminopyridine-pyrrolidine, 6
SMILES: Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCc2ccc(Cl)cc2)c1
InChI Key: InChIKey=JDRSQGJWTVRNGM-QFBILLFUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234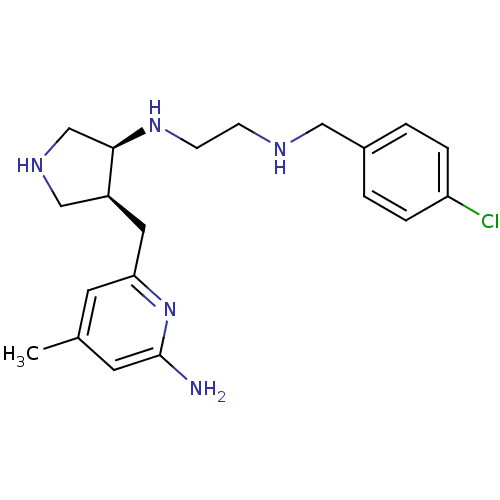 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 85 | -9.80 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS | J Med Chem 53: 7804-24 (2010) Article DOI: 10.1021/jm100947x BindingDB Entry DOI: 10.7270/Q2NC61FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University; The Regents of the University of California US Patent | Assay Description Recombinant NOS isozymes over-expressed in E. coli were utilized. (Ji, H., et al., Discovery of highly potent and selective inhibitors of neuronal ni... | US Patent US9090589 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli by hemoglobin capture assay | J Med Chem 52: 779-97 (2009) Article DOI: 10.1021/jm801220a BindingDB Entry DOI: 10.7270/Q27D2V01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, brain Mutant (D597N/M336V) (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20E+3 | -8.21 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University; The Regents of the University of California US Patent | Assay Description Recombinant NOS isozymes over-expressed in E. coli were utilized. (Ji, H., et al., Discovery of highly potent and selective inhibitors of neuronal ni... | US Patent US9090589 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS | J Med Chem 53: 7804-24 (2010) Article DOI: 10.1021/jm100947x BindingDB Entry DOI: 10.7270/Q2NC61FT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli by hemoglobin capture assay | J Med Chem 52: 779-97 (2009) Article DOI: 10.1021/jm801220a BindingDB Entry DOI: 10.7270/Q27D2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli by hemoglobin capture assay | J Med Chem 52: 779-97 (2009) Article DOI: 10.1021/jm801220a BindingDB Entry DOI: 10.7270/Q27D2V01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS | J Med Chem 53: 7804-24 (2010) Article DOI: 10.1021/jm100947x BindingDB Entry DOI: 10.7270/Q2NC61FT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 8.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University; The Regents of the University of California US Patent | Assay Description Recombinant NOS isozymes over-expressed in E. coli were utilized. (Ji, H., et al., Discovery of highly potent and selective inhibitors of neuronal ni... | US Patent US9090589 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.52E+4 | -5.64 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli by hemoglobin capture assay | Bioorg Med Chem 20: 2435-43 (2012) Article DOI: 10.1016/j.bmc.2012.01.037 BindingDB Entry DOI: 10.7270/Q26110TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat nNOS expressed in HEK293T cells preincubated for 30 mins prior A23187-induced activation measured after 6 hrs by Griess assay | Bioorg Med Chem 20: 2435-43 (2012) Article DOI: 10.1016/j.bmc.2012.01.037 BindingDB Entry DOI: 10.7270/Q26110TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||