

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM300056 N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- methoxypropan-2- yl]oxy}-6-[4-(4- methoxy-1H- pyrrolo[2,3- b]pyridin-3- yl)piperidin-1- yl]pyrimidine-4- carboxamide::US9593097, Example 436::US9593097, Example 437::US9593097, Example 495::US9593097, Example 498::US9593097, Example 499
SMILES: COC[C@@H](C)Oc1nc(cc(n1)C(=O)NC(C)C(C)(C)N)N1CCC(CC1)c1c[nH]c2nccc(OC)c12
InChI Key:
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300056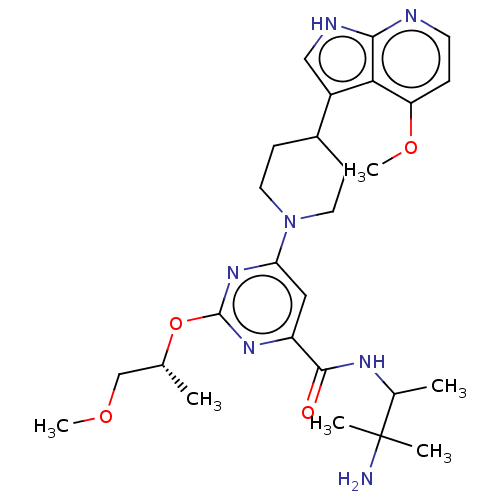 (N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- metho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300056 (N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- metho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300056 (N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- metho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300056 (N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- metho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300056 (N-(3-amino-3- methylbutan-2-yl)- 2-{[(2R)-1- metho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||