

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM30178 Pyrrolopyridine, 9
SMILES: O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1ccccc1
InChI Key: InChIKey=NNQMSHWOTYMSDC-UHFFFAOYSA-N
Data: 6 IC50
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM30178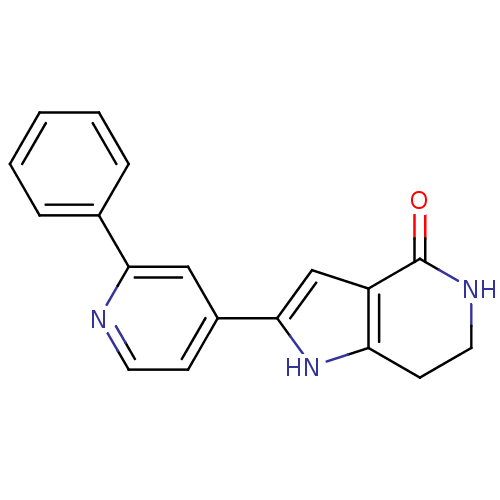 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| MAP kinase-activated protein kinase 3 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Ribosomal protein S6 kinase alpha 4 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha 5 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||