

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCOc1ccc(cc1F)-c1nn([C@H]2C[C@]3(C2)CCN(C3)C(=O)C#CC)c(N)c1C(N)=O
InChI Key: InChIKey=RWTGOZVFNOZKKB-BCTVQRCUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303649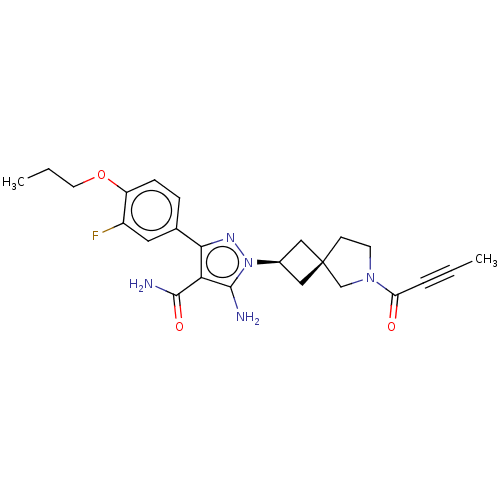 (US10138229, Example 161 | US10875852, Example 161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description An HTRF assay (Cisbio KinEASE-TK cat #62TK0PEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation of ... | US Patent US10875852 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303649 (US10138229, Example 161 | US10875852, Example 161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description TBDAn HTRF assay (Cisbio KinEASE-TK cat #62TKOPEC) was performed to quantitate the ability of test compounds to inhibit BTK mediated phosphorylation ... | US Patent US10138229 (2018) BindingDB Entry DOI: 10.7270/Q2CJ8GJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||