

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCNc1ncc(-c2ncc(CN3CCCC3)s2)c(NC2CC[C@H](O)CC2)n1
InChI Key: InChIKey=DYQYODJNEBRPRK-FITNRVMRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308305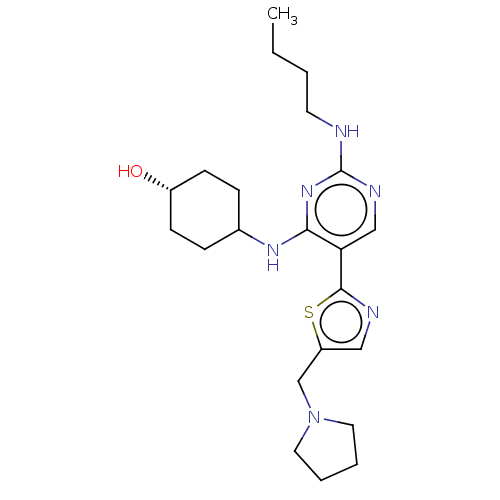 (US9649309, Compound UNC4223A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM308305 (US9649309, Compound UNC4223A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM308305 (US9649309, Compound UNC4223A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth arrest-specific protein 6 (Homo sapiens (Human)) | BDBM308305 (US9649309, Compound UNC4223A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||