

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31132 5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k
SMILES: CCOC(=O)c1c([nH]c2c1cc(O)c1ccccc21)-c1cccc(Cl)c1
InChI Key: InChIKey=ICMUBHJJQVALPP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM31132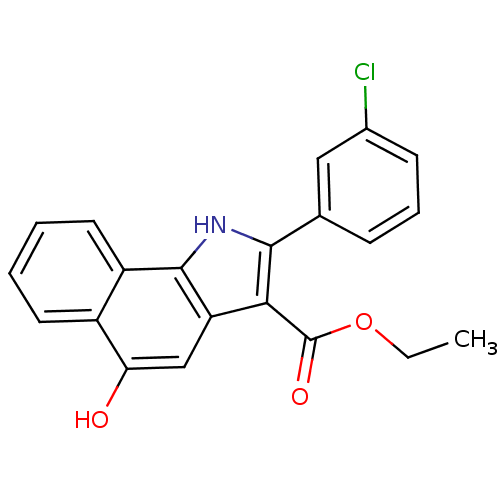 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | 520 | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description For determination of the activity of 5-LO in 40000g supernatant (S40), aliquots of the supernatants were added to reaction mix, and were preincubated... | J Med Chem 52: 3474-83 (2009) Article DOI: 10.1021/jm900212y BindingDB Entry DOI: 10.7270/Q2HM56S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31132 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... | Bioorg Med Chem 23: 4839-45 (2015) BindingDB Entry DOI: 10.7270/Q2M90BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM31132 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli Curated by ChEMBL | Assay Description Inhibition of 5-LO in human peripheral blood neutrophils using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 155: 946-960 (2018) Article DOI: 10.1016/j.ejmech.2018.05.041 BindingDB Entry DOI: 10.7270/Q2WM1H3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM31132 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by PGH2 addition m... | Eur J Med Chem 155: 946-960 (2018) Article DOI: 10.1016/j.ejmech.2018.05.041 BindingDB Entry DOI: 10.7270/Q2WM1H3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM31132 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Campania Luigi Vanvitelli Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins followed by subs... | Eur J Med Chem 155: 946-960 (2018) Article DOI: 10.1016/j.ejmech.2018.05.041 BindingDB Entry DOI: 10.7270/Q2WM1H3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31132 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||