

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31151 3-hydroxyquinolin-2(1H)-one, 5
SMILES: Oc1cc2cc(F)ccc2[nH]c1=O
InChI Key: InChIKey=XMEDGBPWEVERPL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31151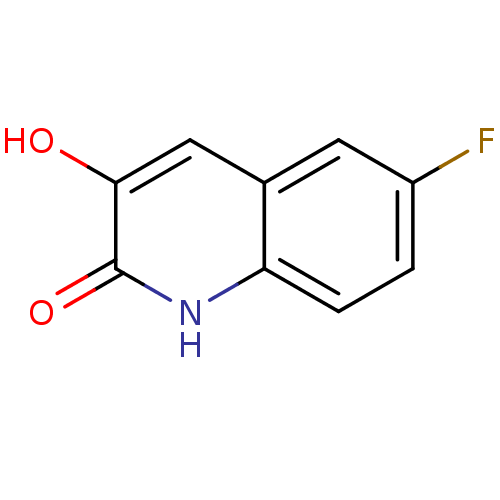 (3-hydroxyquinolin-2(1H)-one, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Rattus norvegicus (rat)) | BDBM31151 (3-hydroxyquinolin-2(1H)-one, 5) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31151 (3-hydroxyquinolin-2(1H)-one, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Binding affinity and kinetics were measured using biotinylated recombinant human DAAO bound to a Neutravidin surface in a Biacore binding assay. A cu... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31151 (3-hydroxyquinolin-2(1H)-one, 5) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >8.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||