

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM312809 1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin-3- yl]ethynyl]phenyl]-6-methoxy- imidazo[1,5-a]pyridine- 3-carboxamide::US9605005, Example 98
SMILES: COc1ccc2c(nc(C(N)=O)n2c1)-c1cccc(c1)C#C[C@]1(O)CCN(C)C1=O
InChI Key: InChIKey=VSFYURZOMRPUHE-QFIPXVFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM312809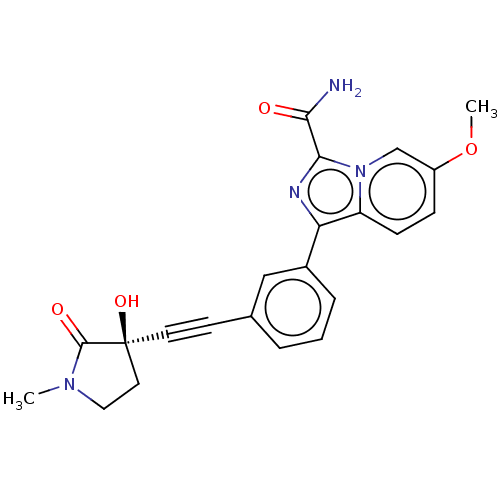 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The ability of the nuclear factor-kappa B (NF-kB)-inducing kinase (NIK) to catalyze the hydrolysis of adenosine-5′-triphosphate (ATP) was monit... | US Patent US9605005 (2017) BindingDB Entry DOI: 10.7270/Q2T43W61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in HEK293 cells harboring NFkB-Luc assessed as reduction in NFkB signal after 24 hrs by luciferase repor... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||