

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3151 2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzene)amido]piperidin-4-yl]oxy}carbonyl)phenyl]carbonyl}-3-hydroxybenzoic acid::Balanol analog 3::CHEMBL67442
SMILES: OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CCNC[C@H]1NC(=O)c1ccc(O)cc1
InChI Key: InChIKey=CLQHLBAJBOQPRR-DYESRHJHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3151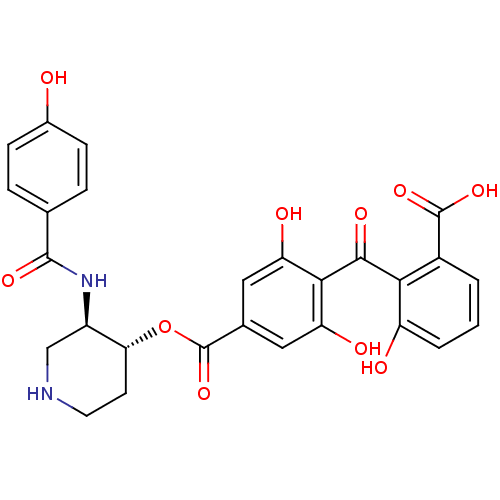 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase (PKA) (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human cAMP-dependent Protein kinase A | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, gamma (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, epsilon (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, eta (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C (PKC) (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-Dependent Protein Kinase (PKA) (Bos taurus (bovine)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphinx Laboratories | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | Bioorg Med Chem Lett 5: 2211-6 (1995) Article DOI: 10.1016/0960-894X(95)00382-4 BindingDB Entry DOI: 10.7270/Q2QR4V9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, eta (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C eta isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C alpha isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C beta 2 isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, epsilon (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C epsilon isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C (PKC) (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C zeta isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C beta 1 isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C delta isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C, gamma (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human Protein kinase C gamma isozyme | J Med Chem 40: 226-35 (1997) Article DOI: 10.1021/jm960497g BindingDB Entry DOI: 10.7270/Q2J965HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM3151 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Sphinx Laboratories | Assay Description PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs. | Bioorg Med Chem Lett 5: 2151-4 (1995) Article DOI: 10.1016/0960-894X(95)00365-Z BindingDB Entry DOI: 10.7270/Q2VH5M1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||