

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31536 pyrazolo[4,3-h]quinazoline-3-carboxamide, 19
SMILES: Cn1nc(C(=O)NC2CCCC2)c2CCc3cnc(Nc4ccccc4)nc3-c12
InChI Key: InChIKey=JBZSHFMYVGCDBI-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-Dependent Kinase 2 (CDK2) (Homo sapiens (Human)) | BDBM31536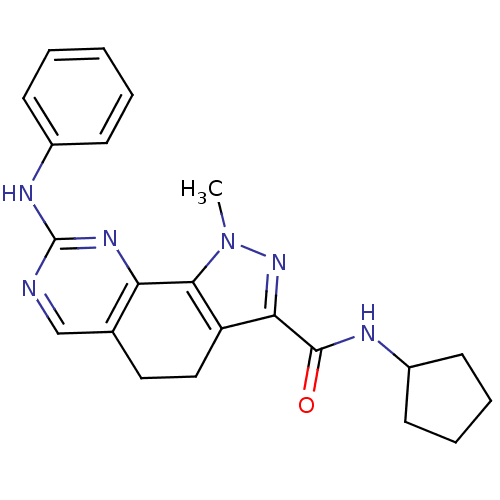 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31536 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31536 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Aurora A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | J Med Chem 53: 3532-51 (2010) Article DOI: 10.1021/jm901713n BindingDB Entry DOI: 10.7270/Q2RF5V5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM31536 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of GST-tagged PLK1 expressed in H5 insect cells assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | J Med Chem 53: 3532-51 (2010) Article DOI: 10.1021/jm901713n BindingDB Entry DOI: 10.7270/Q2RF5V5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK2/Cyclin A/Cyclin A1 (Homo sapiens (Human)) | BDBM31536 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | J Med Chem 53: 3532-51 (2010) Article DOI: 10.1021/jm901713n BindingDB Entry DOI: 10.7270/Q2RF5V5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||