

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM316475 2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- yl)phenyl]carbonyl}piperidin-3- yl]sulfanyl}pyridine-4-carbonitrile::US9617246, 9
SMILES: C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Sc1cc(ccn1)C#N
InChI Key: InChIKey=RUBZNQFRBGEBLL-NVXWUHKLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM316475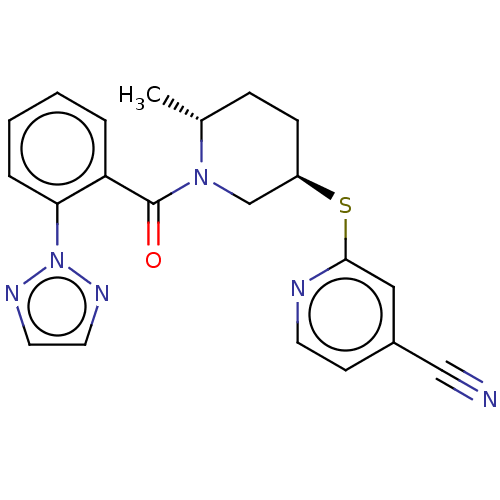 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | Bioorg Med Chem Lett 27: 1364-1370 (2017) BindingDB Entry DOI: 10.7270/Q2WH2S8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM316475 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | Bioorg Med Chem Lett 27: 1364-1370 (2017) BindingDB Entry DOI: 10.7270/Q2WH2S8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM316475 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | US Patent US9617246 (2017) BindingDB Entry DOI: 10.7270/Q2ST7RXW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM316475 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description In a typical experiment the OX1 and OX2 receptor antagonistic activity of the compounds of the present invention was determined in accordance with th... | US Patent US9617246 (2017) BindingDB Entry DOI: 10.7270/Q2ST7RXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM316475 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Antagonist activity at human OX2R expressed in CHOK1 cells assessed as reduction in orexin A-induced calcium flux preincubated followed by orexin A a... | Bioorg Med Chem Lett 27: 1364-1370 (2017) BindingDB Entry DOI: 10.7270/Q2WH2S8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM316475 (2-{[(3R,6R)-6-methyl-1-{[2-(2H- 1,2,3-triazol-2- y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as reduction in orexin A-induced calcium flux preincubated followed by orexin A a... | Bioorg Med Chem Lett 27: 1364-1370 (2017) BindingDB Entry DOI: 10.7270/Q2WH2S8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||