

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM317625 3-(4-{5- Amino-4-[4- (1H-pyrazol-4- yl)-1H-indole- 2-carbonyl]- pyrazol-1-yl}- 3-methyl- phenoxy)- benzonitrile::US10640491, Compound 14::US9624201, Compound 14
SMILES: Cc1cc(Oc2cccc(c2)C#N)ccc1-n1ncc(C(=O)c2cc3c(cccc3[nH]2)-c2cn[nH]c2)c1N
InChI Key: InChIKey=GWSLLIVSORPVSL-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317625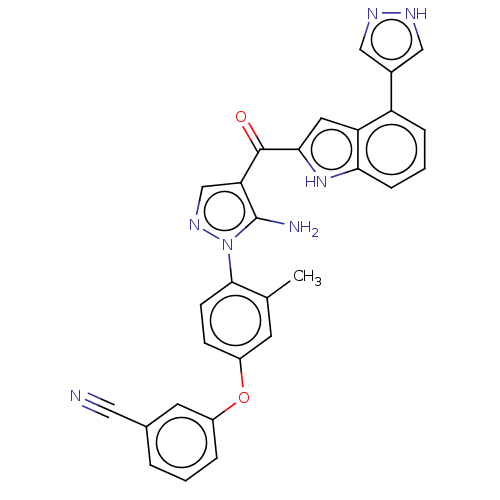 (3-(4-{5- Amino-4-[4- (1H-pyrazol-4- yl)-1H-indole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.38 | n/a | n/a | n/a | n/a | n/a | n/a |
HOFFMANN-LA ROCHE INC.; CHUGAI PHARMACEUTICAL CO. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of Btk, biotinylated SH2 peptide substrate (Src... | US Patent US9624201 (2017) BindingDB Entry DOI: 10.7270/Q2TQ63NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317625 (3-(4-{5- Amino-4-[4- (1H-pyrazol-4- yl)-1H-indole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8.38 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317625 (3-(4-{5- Amino-4-[4- (1H-pyrazol-4- yl)-1H-indole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.38 | n/a | n/a | n/a | n/a | n/a | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640491 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||