

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31828 quinazoline-pyrazolourea hybrid compound, 3b
SMILES: Cc1cccc(c1)-n1nc(cc1NC(=O)Nc1ccc(Nc2ncnc3ccc(N)cc23)cc1)C(C)(C)C
InChI Key: InChIKey=ZKESOLNZMJUNTF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM31828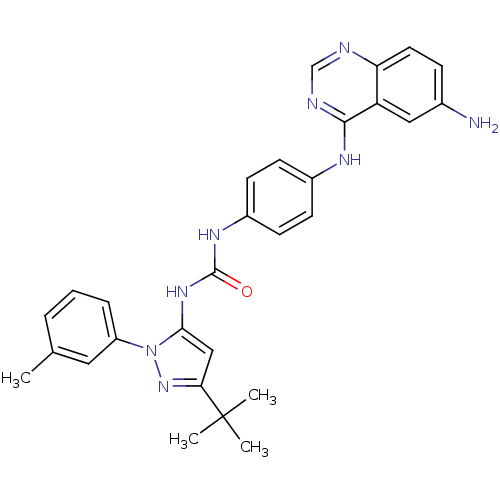 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | 73 | n/a | n/a | n/a | 7.0 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla... | J Med Chem 52: 3915-26 (2009) Article DOI: 10.1021/jm9002928 BindingDB Entry DOI: 10.7270/Q2VT1QD3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Src Mutant (T338M) (Gallus gallus (Chicken)) | BDBM31828 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla... | J Med Chem 52: 3915-26 (2009) Article DOI: 10.1021/jm9002928 BindingDB Entry DOI: 10.7270/Q2VT1QD3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM31828 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Displacemnt of N,N'-(2,2'-(3,3'-disulfanediylbis(2,5-dioxopyrrolidine-3,1-diyl))bis(ethane-2,1-diyl))bis(2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureid... | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM31828 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Displacemnt of N,N'-(2,2'-(3,3'-disulfanediylbis(2,5-dioxopyrrolidine-3,1-diyl))bis(ethane-2,1-diyl))bis(2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureid... | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM31828 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of JNK2 active form by HTRF assay | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM31828 (quinazoline-pyrazolourea hybrid compound, 3b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of p38alpha active form expressed in Escherichia coli BL21(DE3) cells by HTRF assay | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||