

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM319608 3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one::US10174011, Example 3
SMILES: Cc1nc2CCCCn2c(=O)c1CCN1CCN(CC1)c1cccc2sccc12
InChI Key: InChIKey=XMFRRROSDAWYLB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319608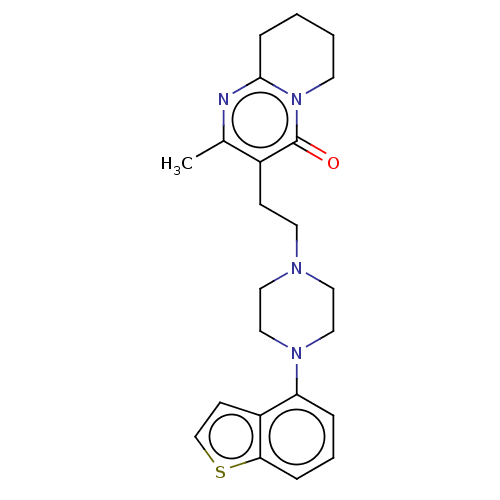 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.57 | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT1A receptor agonism activity test (The agonism activity of test compounds on 5-HT1A receptor expressing human recombinant 5-HT1A receptor in ... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.61 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The D2 receptor antagonism activity test (The antagonism activity of test compounds on D2 receptor expressing human recombinant D2 receptor in HEK293... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES; SUZHOU VIGONVITA LIFE SCIENCES CO., LTD.; TOPHARMAN SHANDONG CO., LTD. US Patent | Assay Description The 5-HT2A receptor antagonism activity test (The antagonism activity of test compounds on 5-HT2A receptor expressing human recombinant 5-HT2A recept... | US Patent US10174011 (2019) BindingDB Entry DOI: 10.7270/Q2VH5QX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2A receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor (unknown origin) after 60 mins by Ultra lance cAMP assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Agonist activity at 5-HT1A receptor (unknown origin) after 60 mins by Ultra lance cAMP assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM319608 (3-(2-(4-(benzo[b]thiophen-4-yl)piperazin-1-yl)ethy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Xinjiang Technical Institute of Physics and Chemistry Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2C receptor (unknown origin) after 10 mins by calcium 5 dye based FLIPR assay | Bioorg Med Chem 25: 4904-4916 (2017) Article DOI: 10.1016/j.bmc.2017.07.040 BindingDB Entry DOI: 10.7270/Q2Z3222T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||