

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3303 4-Anilinoquinazoline deriv. 54::4-N-(3-bromophenyl)quinazoline-4,6,7-triamine::4[ (3-Bromophenyl)aminol-6,7-diaminoquinazoline::CHEMBL328216
SMILES: Nc1cc2ncnc(Nc3cccc(Br)c3)c2cc1N
InChI Key: InChIKey=ADXSZLCTQCWMTE-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303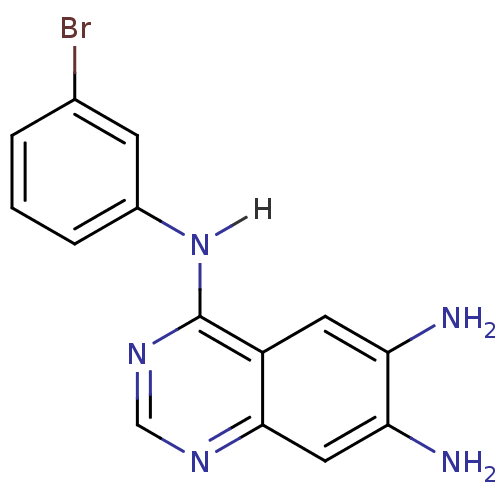 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 38: 3482-7 (1995) Article DOI: 10.1021/jm00018a008 BindingDB Entry DOI: 10.7270/Q2319T3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of wild type EGFR | Proc Natl Acad Sci USA 104: 20523-8 (2007) Checked by Author Article DOI: 10.1073/pnas.0708800104 BindingDB Entry DOI: 10.7270/Q2DB82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem 18: 6634-45 (2010) Article DOI: 10.1016/j.bmc.2010.08.004 BindingDB Entry DOI: 10.7270/Q25H7GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of EGFR autophosphorylation in human A431 cells | Bioorg Med Chem 18: 6634-45 (2010) Article DOI: 10.1016/j.bmc.2010.08.004 BindingDB Entry DOI: 10.7270/Q25H7GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Inhibition of EGFR | J Med Chem 51: 1179-88 (2008) Article DOI: 10.1021/jm070654j BindingDB Entry DOI: 10.7270/Q29Z95RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||