

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM33267 azo-sulfonamide, 1b
SMILES: Nc1ccc(cc1)\N=N\c1ccc(cc1)S(N)(=O)=O
InChI Key: InChIKey=JSHXITVMUADQEA-FOCLMDBBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM33267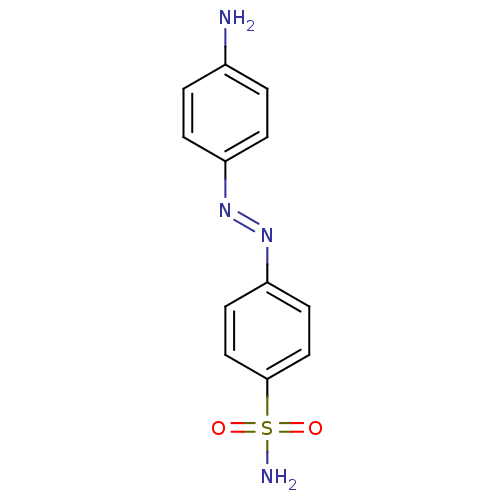 (azo-sulfonamide, 1b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 17: 7093-9 (2009) Article DOI: 10.1016/j.bmc.2009.09.003 BindingDB Entry DOI: 10.7270/Q2M32T4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM33267 (azo-sulfonamide, 1b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 17: 7093-9 (2009) Article DOI: 10.1016/j.bmc.2009.09.003 BindingDB Entry DOI: 10.7270/Q2M32T4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM33267 (azo-sulfonamide, 1b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 95 | -9.57 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 17: 7093-9 (2009) Article DOI: 10.1016/j.bmc.2009.09.003 BindingDB Entry DOI: 10.7270/Q2M32T4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM33267 (azo-sulfonamide, 1b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human cloned full length carbonic anhydrase 2 by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4929-32 (2009) Article DOI: 10.1016/j.bmcl.2009.07.088 BindingDB Entry DOI: 10.7270/Q2H70GSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM33267 (azo-sulfonamide, 1b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 106 | -9.51 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 17: 7093-9 (2009) Article DOI: 10.1016/j.bmc.2009.09.003 BindingDB Entry DOI: 10.7270/Q2M32T4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase (mtCA 1) (Mycobacterium tuberculosis) | BDBM33267 (azo-sulfonamide, 1b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4929-32 (2009) Article DOI: 10.1016/j.bmcl.2009.07.088 BindingDB Entry DOI: 10.7270/Q2H70GSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PROBABLE TRANSMEMBRANE CARBONIC ANHYDRASE (CARBONATE DEHYDRATASE) (CARBONIC DEHYDRATASE) (Mycobacterium tuberculosis) | BDBM33267 (azo-sulfonamide, 1b) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv3273 by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4929-32 (2009) Article DOI: 10.1016/j.bmcl.2009.07.088 BindingDB Entry DOI: 10.7270/Q2H70GSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||