 Found 18 hits for monomerid = 33279
Found 18 hits for monomerid = 33279 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33279
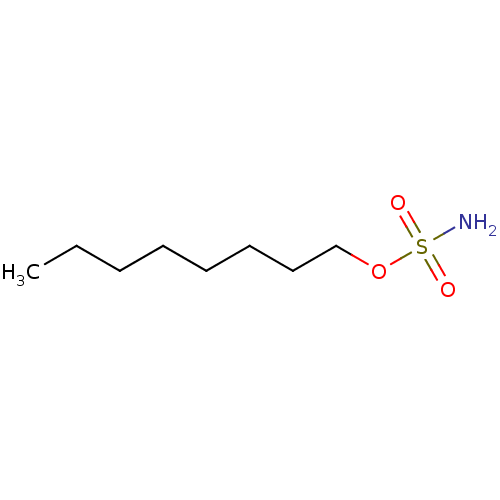
(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.60 | -11.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 46: 2197-204 (2003)
Article DOI: 10.1021/jm021124k
BindingDB Entry DOI: 10.7270/Q28P617T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase I |
J Med Chem 46: 2197-204 (2003)
Article DOI: 10.1021/jm021124k
BindingDB Entry DOI: 10.7270/Q28P617T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | -11.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory concentration against catalytic domain of human cloned carbonic anhydrase IX. |
J Med Chem 46: 2197-204 (2003)
Article DOI: 10.1021/jm021124k
BindingDB Entry DOI: 10.7270/Q28P617T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 76.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 79.9 | -9.61 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |
Carbonic Anhydrase XIV
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic Anhydrase VB
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6 (CA-VI)
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic Anhydrase XIII
(Mus musculus (mouse)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic Anhydrase VA
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+3 | -7.99 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic Anhydrase III
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.10E+3 | -6.83 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM33279

(CHEMBL24261 | aliphatic sulfamate, 1)Show InChI InChI=1S/C8H19NO3S/c1-2-3-4-5-6-7-8-12-13(9,10)11/h2-8H2,1H3,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data