

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM335434 (3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- methoxy-1,7-naphthyridin-8- yl)amino)pyridin-4-yl)-3,6- dimethyl-3,6-dihydro-2H-1,4- thiazine 1,1-dioxide::US9732088, Example 6
SMILES: COc1cnc2c(Nc3cc(c(F)cn3)[C@]3(C)CS(=O)(=O)[C@@](C)(C4CC4)C(N)=N3)nccc2c1
InChI Key: InChIKey=CVIKZJGUFZXXGR-GOTSBHOMSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM335434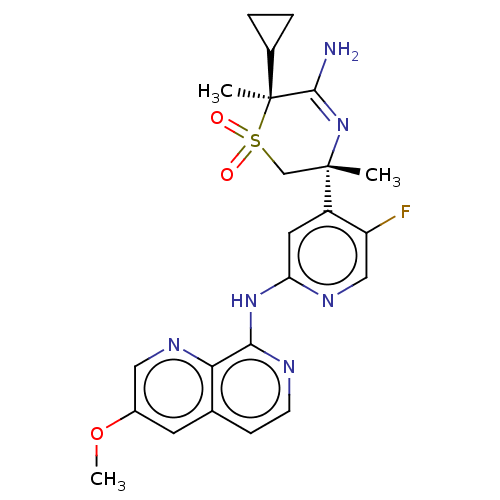 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-2 using the following assay. Inhibitor IC50s at purified human autoBAC... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335434 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM335434 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The following reagents were used in this assay: Na+-Acetate pH 5.0; 1% Brij-35; Dimethyl Sulfoxide (DMSO); Purified human Cathepsin-D (>95% pure); As... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||