

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [O-][N+](=O)c1ccccc1-c1nc2ccccc2c(=O)o1
InChI Key: InChIKey=DWCFIKGNUJGZBZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM33703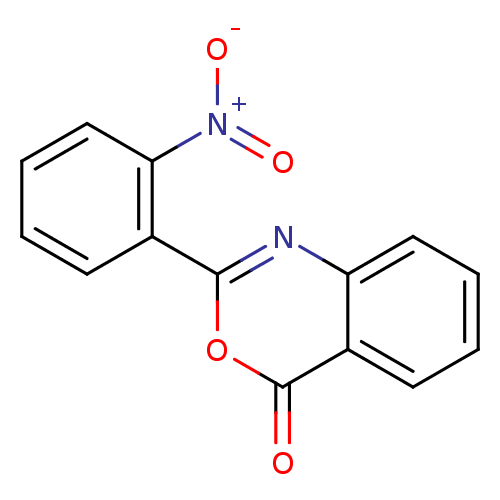 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Molecular Library Screening Center Network (MLSCN) Penn Center for Molecular Discovery (PCMD) Assay Provider: Scott L. Diamond, University of Pennsy... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21G0JQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | 7.6 | n/a |
University of Karachi | Assay Description The α-chymotrypsin inhibition activity was evaluated in 50 mM Tris-HCl buffer pH 7.6 with 10 mM CaCl2. α-Chymotrypsin (bovine pancreas) at ... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against C1r serine protease in assay 1 | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article | n/a | n/a | >6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against C1r serine protease . | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM33703 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PCBioAssay | n/a | n/a | 116 | n/a | n/a | n/a | n/a | 7.5 | 23 |
PCMD Curated by PubChem BioAssay | Assay Description HTS was performed using 217,350 compounds of the MLSCN library individually plated into 10ul 1536 compound plates at a concentration of 2.5 mM each, ... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K64GDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||