

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM339883 US9757383, Example 6::trans-4-[{2-[{[4-({[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethyl]amino}methyl)phenoxy]acetyl}(methyl)amino]ethyl}(methyl)amino]cyclohexyl hydroxy(di-2-thienyl)acetate
SMILES: CN(CCN(C)C(=O)COc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1)[C@H]1CC[C@@H](CC1)OC(=O)C(O)(c1cccs1)c1cccs1
InChI Key: InChIKey=LICFTXXXFSVXLE-GSZYCOFVSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM339883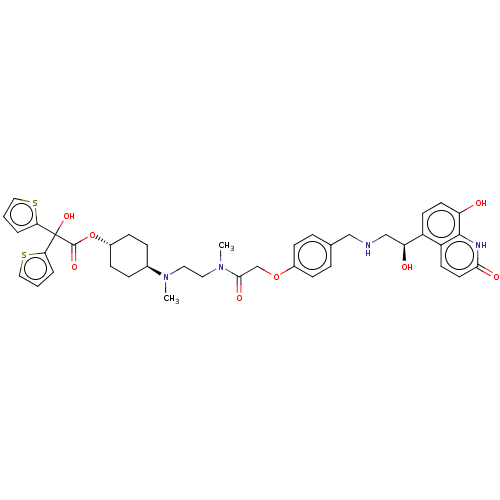 (US9757383, Example 6 | trans-4-[{2-[{[4-({[(2R)-2-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9757383 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM339883 (US9757383, Example 6 | trans-4-[{2-[{[4-({[(2R)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9757383 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||