

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3414 (2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-3-hydroxy-1-phenylbutan-2-yl]-2-(quinolin-2-ylformamido)butanediamide::LY289612
SMILES: CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1
InChI Key: InChIKey=RHADMHOBZRGRTJ-OIFRRMEBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM3414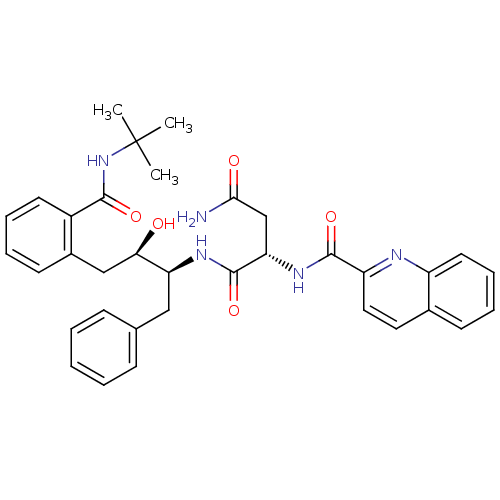 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -12.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human immunodeficiency virus protease (HIVP) using protease inhibition assay | Bioorg Med Chem Lett 5: 721-726 (1995) Article DOI: 10.1016/0960-894X(95)00102-Y BindingDB Entry DOI: 10.7270/Q2FJ2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound was tested for inhibitory activity against isolated HIV-1 protease | Citation and Details Article DOI: 10.1016/S0960-894X(01)80186-4 BindingDB Entry DOI: 10.7270/Q2W66PGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 1391-1396 (1994) Article DOI: 10.1016/S0960-894X(01)80368-1 BindingDB Entry DOI: 10.7270/Q2KS6RGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||