

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM342911 1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,2,4-oxadiazol-3-yl-6-isoquinolinesulfonamide::US9776995, Example 7
SMILES: COc1cc(c(Cl)cc1-c1nccc2cc(ccc12)S(=O)(=O)Nc1ncon1)-c1cccc(F)c1
InChI Key: InChIKey=RPJWVSTULWOXEQ-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM342911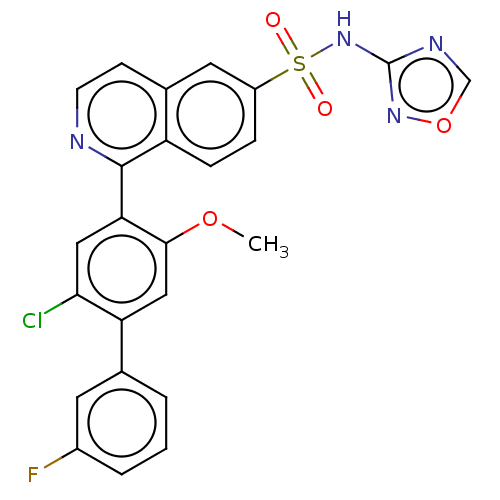 (1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. US Patent | Assay Description HEK 293 cells stably transfected with human Nav1.7 were recorded in whole cell voltage clamp mode with the PatchXpress automated electrophysiology sy... | US Patent US9776995 (2017) BindingDB Entry DOI: 10.7270/Q2Q81G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM342911 (1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. US Patent | Assay Description 293 cells stably transfected with Nav 1.5 were recorded in whole cell voltage clamp mode with the PatchXpress automated electrophysiology system acco... | US Patent US9776995 (2017) BindingDB Entry DOI: 10.7270/Q2Q81G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM342911 (1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human Glucocorticoid receptor (GR); Value ranges from 378-814 nM | J Med Chem 60: 5969-5989 (2017) Article DOI: 10.1021/acs.jmedchem.6b01851 BindingDB Entry DOI: 10.7270/Q2RJ4MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM342911 (1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 5969-5989 (2017) Article DOI: 10.1021/acs.jmedchem.6b01851 BindingDB Entry DOI: 10.7270/Q2RJ4MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM342911 (1-(2-chloro-3'-fluoro-5-methoxy-4-biphenylyl)-N-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells incubated for 3 to 5 mins at -125 mV holding potential by electrophysiology assay | J Med Chem 60: 5969-5989 (2017) Article DOI: 10.1021/acs.jmedchem.6b01851 BindingDB Entry DOI: 10.7270/Q2RJ4MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||