

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM35025 benzothienopyrimidinone deriv., 14j
SMILES: CN(C)Cc1nc2c3cc(ccc3sc2c(=O)[nH]1)-c1ccc(O)cc1
InChI Key: InChIKey=PJNOYUGDEFSKQL-UHFFFAOYSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM35025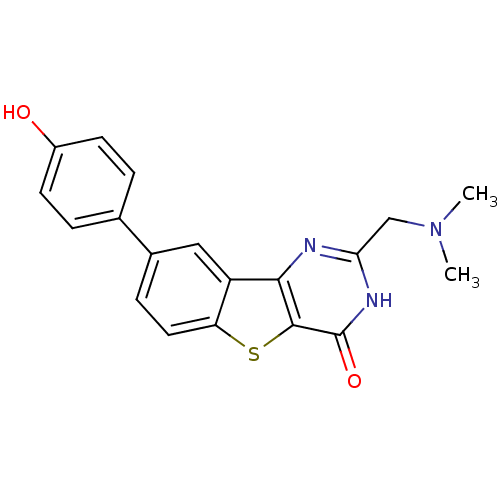 (benzothienopyrimidinone deriv., 14j) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -11.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM35025 (benzothienopyrimidinone deriv., 14j) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -11.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||