 Found 6 hits for monomerid = 35346
Found 6 hits for monomerid = 35346 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen Phosphorylase (PYGL)
(Homo sapiens (Human)) | BDBM35346
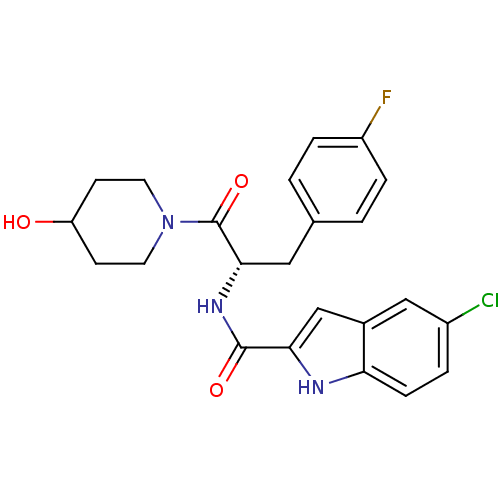
((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen Phosphorylase (PYGL)
(Homo sapiens (Human)) | BDBM35346

((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |
Sterol 14α-demethylase (CYP51)
(Homo sapiens (Human)) | BDBM35346

((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ACT LLC
Curated by ChEMBL
| Assay Description
Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assay |
Drug Metab Dispos 35: 493-500 (2007)
Article DOI: 10.1124/dmd.106.013888
BindingDB Entry DOI: 10.7270/Q2DF6S2H |
More data for this
Ligand-Target Pair | |
Glycogen Phosphorylase (PYGL)
(Homo sapiens (Human)) | BDBM35346

((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of pig liver Glycogen phosphorylase A. |
J Med Chem 47: 3537-45 (2004)
Article DOI: 10.1021/jm031121n
BindingDB Entry DOI: 10.7270/Q2HM5977 |
More data for this
Ligand-Target Pair | |
Muscle glycogen phosphorylase
(Homo sapiens (Human)) | BDBM35346

((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of Rabbit muscle glycogen phosphorylase B. |
J Med Chem 47: 3537-45 (2004)
Article DOI: 10.1021/jm031121n
BindingDB Entry DOI: 10.7270/Q2HM5977 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen Phosphorylase (PYGL)
(Homo sapiens (Human)) | BDBM35346

((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data