

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3570 8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazoline::Anilinopyrimidine deriv. 7a::CHEMBL66031::Imidazoquinazoline deriv. 8::N-(3-bromophenyl)-1H-imidazo[4,5-g]quinazolin-8-amine
SMILES: Brc1cccc(Nc2ncnc3cc4nc[nH]c4cc23)c1
InChI Key: InChIKey=BZHDPIVTQKXAQQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3570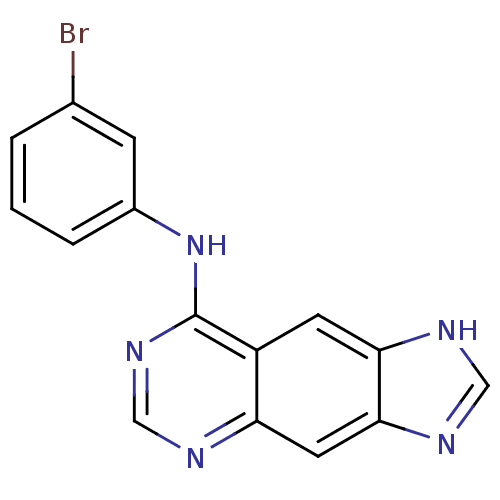 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3570 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (FBPase) (Homo sapiens (Human)) | BDBM3570 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Concentration required to inhibit the human liver recombinant fructose-1,6-bisphosphatase. | Bioorg Med Chem Lett 11: 17-21 (2001) BindingDB Entry DOI: 10.7270/Q2HD7W52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 2 (Homo sapiens (Human)) | BDBM3570 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc. Curated by ChEMBL | Assay Description Inhibition of EPH receptor B2 using ELISA | J Med Chem 48: 3221-30 (2005) Article DOI: 10.1021/jm0492204 BindingDB Entry DOI: 10.7270/Q2DF6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM3570 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of MNK1 | J Med Chem 53: 6618-28 (2010) Article DOI: 10.1021/jm1005513 BindingDB Entry DOI: 10.7270/Q2Z038CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 2 (Homo sapiens (Human)) | BDBM3570 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a |
Protana Inc. Curated by ChEMBL | Assay Description Equilibrium binding constant for EPH receptor B2 | J Med Chem 48: 3221-30 (2005) Article DOI: 10.1021/jm0492204 BindingDB Entry DOI: 10.7270/Q2DF6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||