

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM358657 (S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-(5- ethyl-2,2-dimethyl-3-oxomorpholino) benzonitrile::US10214537, Example 734::US10214537, Example 735
SMILES: CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F
InChI Key: InChIKey=GGLBMZQGKMGJKM-AWEZNQCLSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM358657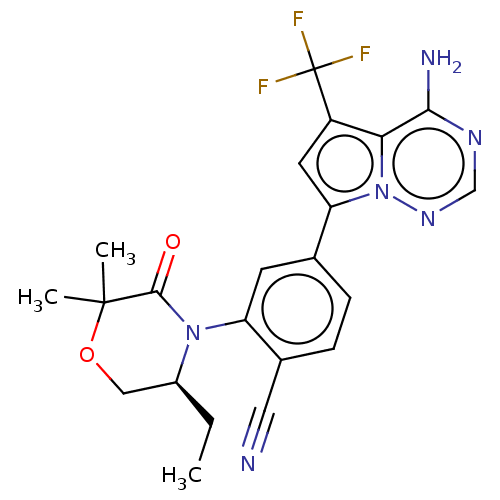 ((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... | US Patent US10214537 (2019) BindingDB Entry DOI: 10.7270/Q2HH6NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM358657 ((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... | US Patent US10214537 (2019) BindingDB Entry DOI: 10.7270/Q2HH6NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM358657 ((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay | Bioorg Med Chem Lett 27: 2849-2853 (2017) Article DOI: 10.1016/j.bmcl.2017.01.077 BindingDB Entry DOI: 10.7270/Q25M684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM358657 ((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in ACD-treated human whole blood assessed as reduction in CD69 expression measured after 1 hr | Bioorg Med Chem Lett 27: 2849-2853 (2017) Article DOI: 10.1016/j.bmcl.2017.01.077 BindingDB Entry DOI: 10.7270/Q25M684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM358657 ((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in ACD-treated human whole blood assessed as reduction in IFNgammaX production measured after 1 hr | Bioorg Med Chem Lett 27: 2849-2853 (2017) Article DOI: 10.1016/j.bmcl.2017.01.077 BindingDB Entry DOI: 10.7270/Q25M684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||