

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM362044 US10272077, Example 4::US9833448, Example 4
SMILES: C[C@@H]1[C@@H](C[C@H](NC(=O)c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)N1CC(F)(F)F)c1c(F)ccc(F)c1F
InChI Key: InChIKey=QIVUCLWGARAQIO-OLIXTKCUSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin receptor-like receptor (CLR) (Homo sapiens (Human)) | BDBM362044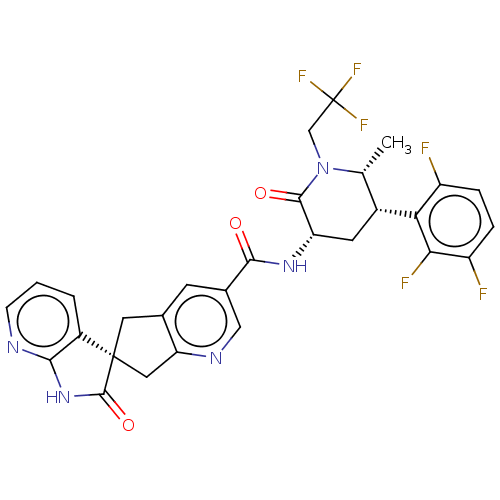 (US10272077, Example 4 | US9833448, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM362044 (US10272077, Example 4 | US9833448, Example 4) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase UniChem | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biohaven Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CLR/RAMP1 | J Med Chem 63: 6600-6623 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CGRP type 1 receptor (Homo sapiens (Human)) | BDBM362044 (US10272077, Example 4 | US9833448, Example 4) | PDB GoogleScholar AffyNet | Purchase UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||