

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCS(=O)(=O)c1ccc(cc1)[C@H](CO)NC(=O)c1ccc2[C@H](C(C)C)N(C[C@H]3CC[C@@H](CC3)C(F)(F)F)Cc2c1
InChI Key: InChIKey=MMBPHZSLRCFGSR-ZDPZMXHYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM362862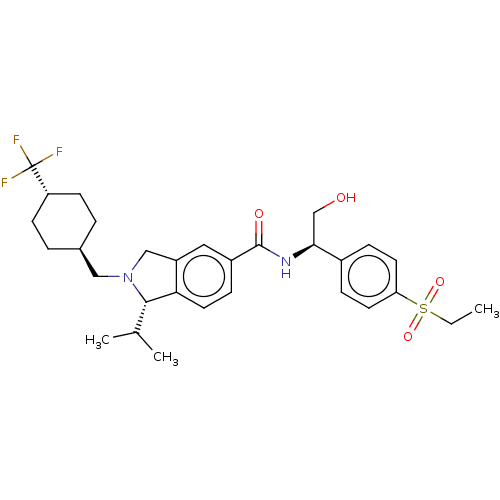 (US9845308, Example 3a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the present invention were tested for ability to bind to RORγ in a cell-free competition assay with commercially available radio-li... | US Patent US9845308 (2017) BindingDB Entry DOI: 10.7270/Q2DF6TG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||