 Found 4 hits for monomerid = 36372
Found 4 hits for monomerid = 36372 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372
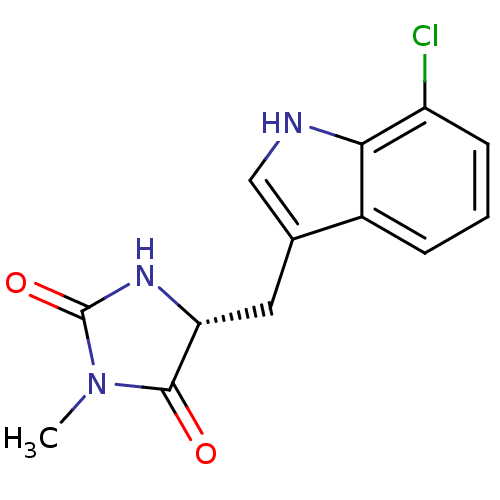
((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | 7.3 | 30 |
Tufts University
| Assay Description
In vitro kinase assay using RIP1 |
Nat Chem Biol 4: 313-21 (2008)
Article DOI: 10.1038/nchembio.83
BindingDB Entry DOI: 10.7270/Q21V5C91 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372

((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 in human U937 cells assessed as inhibition of TNF/zVAD.fmk induced necroptosis after 24 hrs by Cell titer-Glo luminescence a... |
J Med Chem 59: 2163-78 (2016)
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372

((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) after 4 hrs by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM36372

((5R)-5-[(7-chloro-1H-indol-3-yl)methyl]-3-methyl-2...)Show InChI InChI=1S/C13H12ClN3O2/c1-17-12(18)10(16-13(17)19)5-7-6-15-11-8(7)3-2-4-9(11)14/h2-4,6,10,15H,5H2,1H3,(H,16,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data