

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM36486 9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenylamino)-5-methyl-8,9-dihydro-5H-pyrimido[5,4-b][1,4]diazepin-6(7H)-one::Mps1-IN-2::Scaffold, B13::US9266890, I-10
SMILES: CCOc1cc(ccc1Nc1ncc2N(C)C(=O)CCN(C3CCCC3)c2n1)N1CCC(O)CC1
InChI Key: InChIKey=WELBJLUKWAJOQV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM36486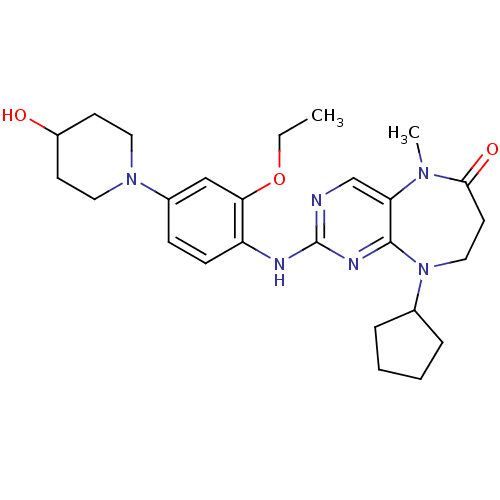 (9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Dana-Farber Cancer Institute | Assay Description ATP-site competition binding assay using Mps1 Kinase inhibitors in LanthaScreen Eu time-resolved FRET (TR-FRET) technology from Invitrogen. | Nat Chem Biol 6: 359-68 (2010) Article DOI: 10.1038/nchembio.345 BindingDB Entry DOI: 10.7270/Q27D2SGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM36486 (9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro Plk1 binding assay: Ambit Kd values in nanomolar. Kd values generated by Ambit binding assay over a concentration range of the compound. | US Patent US9266890 (2016) BindingDB Entry DOI: 10.7270/Q27M06RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM36486 (9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | 25 |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description Kinase reactions were carried out at room temperature with the following components: 1× kinase reaction buffer, 5 μg/mL (40 nM) Mps1 kinase, 200... | US Patent US9266890 (2016) BindingDB Entry DOI: 10.7270/Q27M06RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM36486 (9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | 26 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||