

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1(C)C(=O)C(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O
InChI Key: InChIKey=KFBPBWUZXBYJDG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor alpha [200-419]/gamma [183-417] (Homo sapiens (Human)) | BDBM36810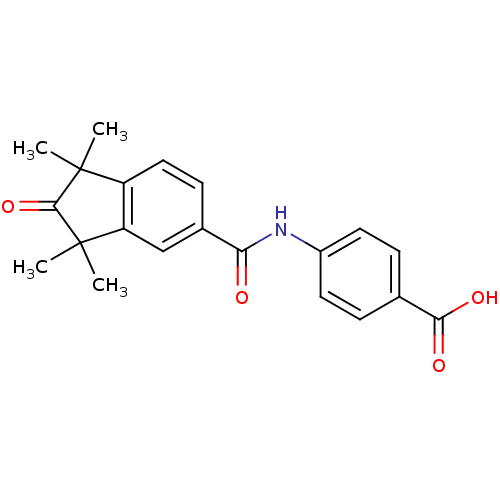 (BMS753 | US9963439, BMS753) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -11.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) | Assay Description Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... | Chem Biol 6: 519-29 (1999) Article DOI: 10.1016/S1074-5521(99)80084-2 BindingDB Entry DOI: 10.7270/Q2CV4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha [200-419] (Homo sapiens (Human)) | BDBM36810 (BMS753 | US9963439, BMS753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -11.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 4 |
Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) | Assay Description Competitive assay were perform with 5nM tritiated all-trans retinoic acid (t-RA; 5nM) with or without 100-fold excess of non-radioactive t-RA (500nM)... | Chem Biol 6: 519-29 (1999) Article DOI: 10.1016/S1074-5521(99)80084-2 BindingDB Entry DOI: 10.7270/Q2CV4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM36810 (BMS753 | US9963439, BMS753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM36810 (BMS753 | US9963439, BMS753) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||