

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM369555 US10231967, Example 68-Isomer-1
SMILES: CCC1=N[C@@]2(N=C1N)c1cc(ccc1C[C@@]21CC[C@@H](CC1)OC)-c1cncc(c1)C#CC
InChI Key: InChIKey=CGJQMXWLLKPNOI-GDKKVTFASA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM369555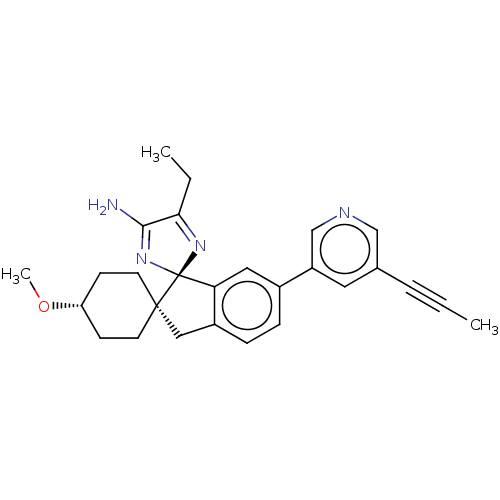 (US10231967, Example 68-Isomer-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description SH-SY5Y cells were cultured in DMEM/F-12 with Glutamax, 10% FCS and 1% non-essential amino acids and cryopreserved and stored at −140° C. at a ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2RF5X91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM369555 (US10231967, Example 68-Isomer-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The β-secretase enzyme used in the TR-FRET is prepared as follows: The cDNA for the soluble part of the human β-Secretase (AA 1-AA 460) was... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2RF5X91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||