

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM370195 US10233188, Example 82::US10800783, Example 82
SMILES: Cn1cc(cn1)S(=O)(=O)N1CCC(CC1)Nc1ncc2ccc(=O)n([C@@H]3CCC[C@@]3(C)O)c2n1
InChI Key: InChIKey=GPNPQTRGIAJGBJ-XMSQKQJNSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDK2/CycE (Homo sapiens (Human)) | BDBM370195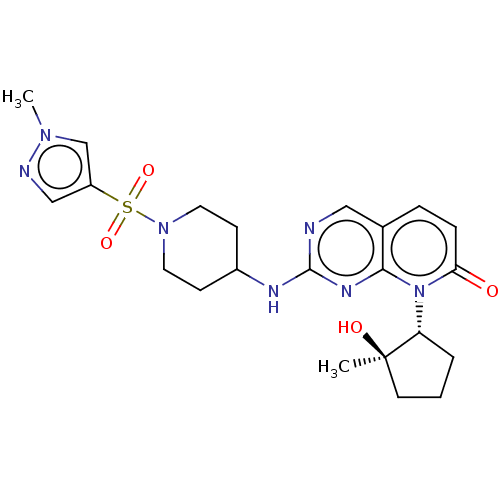 (US10233188, Example 82 | US10800783, Example 82) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK2/CycE (Homo sapiens (Human)) | BDBM370195 (US10233188, Example 82 | US10800783, Example 82) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK6/Cyclin D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370195 (US10233188, Example 82 | US10800783, Example 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK6/Cyclin D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370195 (US10233188, Example 82 | US10800783, Example 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||