

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: N(c1ccccc1)c1ncc2[nH]c(cc2n1)-c1ccccc1
InChI Key: InChIKey=YHCGTIVCLOQBAU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM372148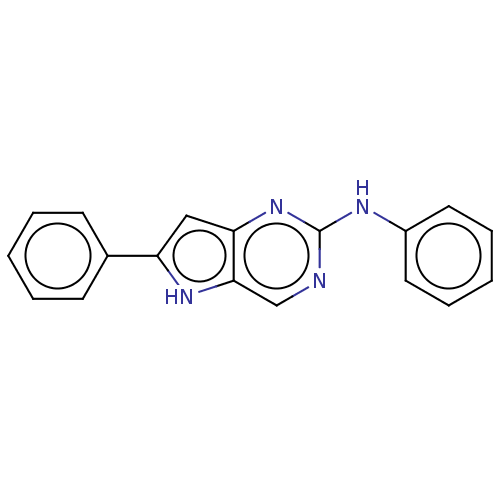 (N,6-diphenyl-5H- pyrrolo[3,2- d]pyrimidin-2-amine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY HEALTH NETWORK US Patent | Assay Description Active RIPK2 was purchased from Life Technologies as His-tagged of catalytic domain (amin acids 1-299) of human RIPK2 kinase expressed in insect cell... | US Patent US10875863 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM372148 (N,6-diphenyl-5H- pyrrolo[3,2- d]pyrimidin-2-amine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description RIPK2 activity was measured using an indirect ELISA detection system. His—RIPK2 (0.6 nM) was incubated in the presence of 6 μM ATP (Sigma cat # ... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q289186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||