

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM372344 Preparation of diasteromeric (1R)- and (1S)-4-((1R,3aS,5aR,5bR,7aR,11aS,11bR,13aR,13bR)-3a-((2-(1,1-dioxidothiomorpholino)ethyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl)-1-(fluoromethyl)cyclohex-3-enecarboxylic acid::US10245275, Example 2
SMILES: CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC=C(C6=CCC(CF)(CC6)C(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)NCCN1CCS(=O)(=O)CC1
InChI Key: InChIKey=YFSNREBZTKMFEB-YJMSPERESA-N
Data: 2 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 gap A364V (Human immunodeficiency virus type 1 group M subtyp...) | BDBM372344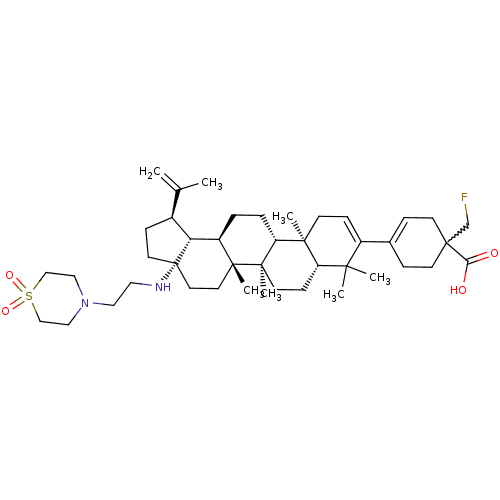 (Preparation of diasteromeric (1R)- and (1S)-4-((1R...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 84.7 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description MT-2 cells and 293T cells were obtained from the NIH AIDS Research and Reference Reagent Program. MT-2 cells were propagated in RPMI 1640 media suppl... | Bioorg Med Chem Lett 15: 1435-40 (2005) BindingDB Entry DOI: 10.7270/Q2MK6G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Capsid Protein) (Human immunodeficiency virus type 1 group M subtyp...) | BDBM372344 (Preparation of diasteromeric (1R)- and (1S)-4-((1R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description MT-2 cells and 293T cells were obtained from the NIH AIDS Research and Reference Reagent Program. MT-2 cells were propagated in RPMI 1640 media suppl... | Bioorg Med Chem Lett 15: 1435-40 (2005) BindingDB Entry DOI: 10.7270/Q2MK6G6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||