

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)C1CCCN(C1)C(=O)\C=C\C(F)F
InChI Key: InChIKey=KSNRPWDSUMXQLE-MDZDMXLPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850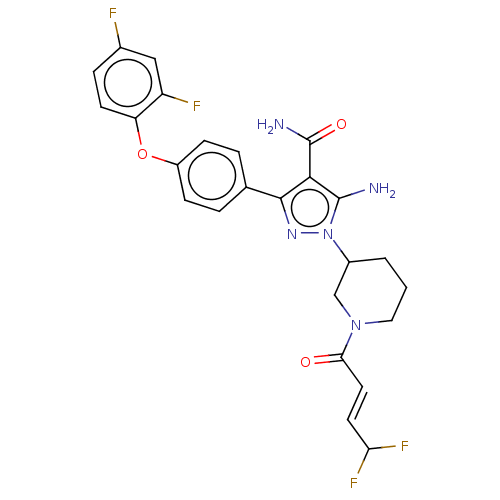 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM377850 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description EGFR: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 20 μM ATP, 100 nM peptide subs... | US Patent US10815213 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM377850 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 20 μM ATP, 100 nM peptide substrate ... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||