

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3789 1-Phenylbenzimidazole Analog 6::2-phenyl-1H-1,3-benzodiazole::US9138393, 2-Phenyl benzimidazole::US9144538, 2-Phenyl benzimidazole::cid_12855
SMILES: c1ccc(cc1)-c1nc2ccccc2[nH]1
InChI Key: InChIKey=DWYHDSLIWMUSOO-UHFFFAOYSA-N
PDB links: 88 PDB IDs contain this monomer as substructures. 88 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM3789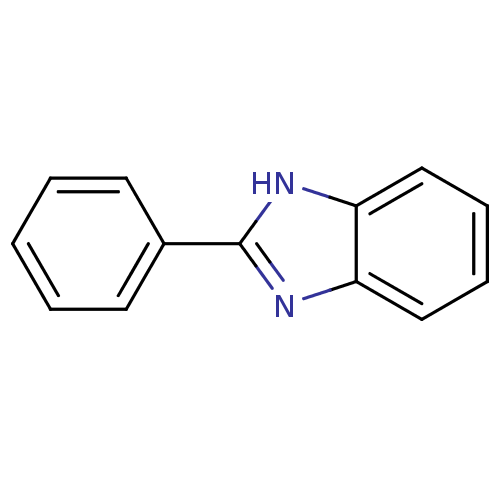 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 5457-65 (1998) Article DOI: 10.1021/jm9804681 BindingDB Entry DOI: 10.7270/Q24T6GJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3789 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 5457-65 (1998) Article DOI: 10.1021/jm9804681 BindingDB Entry DOI: 10.7270/Q24T6GJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM3789 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM3789 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ... | US Patent US9138393 (2015) BindingDB Entry DOI: 10.7270/Q2GF0S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM3789 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ... | US Patent US9144538 (2015) BindingDB Entry DOI: 10.7270/Q22806DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hsf1 protein (Mus musculus) | BDBM3789 (1-Phenylbenzimidazole Analog 6 | 2-phenyl-1H-1,3-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.95E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Modified NIH3T3, transformed to express firefly... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2MW2FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||