

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM4144 (2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2,5-dimethylphenyl)formamido]-4-phenylbutanoyl]pyrrolidine-2-carboxamide::(3 -Hydroxy- 2,5 -dimethylbenzoyl) - (2(S) -hydroxy-3(S)-amino-4-phenylbutanoyl)-4(S)-Cl-Pro-NH-t-Bu::AHPBA deriv. 29::CHEMBL113665
SMILES: Cc1cc(O)c(C)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1C[C@@H](Cl)C[C@H]1C(=O)NC(C)(C)C
InChI Key: InChIKey=FAEDRMSLDDNVCR-CHLMOITFSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM4144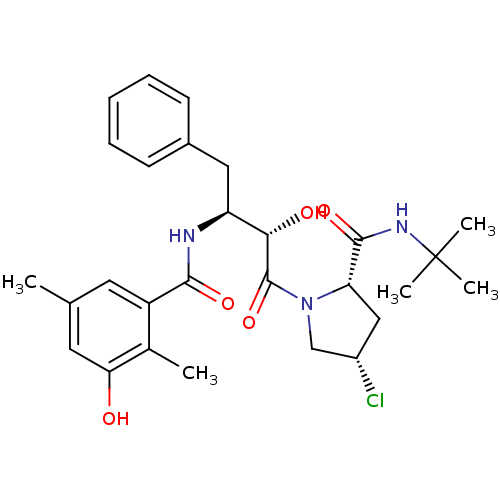 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 333-43 (2002) BindingDB Entry DOI: 10.7270/Q20K27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM4144 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||