

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM4236 (2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)-4-chloropyrrolidin-1-yl]-3-hydroxy-4-oxo-1-phenylbutan-2-yl]-2-[(7-methoxy-1-benzofuran-2-yl)formamido]butanediamide::(7-Methoxybenzofuran-2-carbonyl)-Asn-(2S,3S)-AHPBA-[4(S)-chloro]Pro tert-butylamide::AHPBA 44a
SMILES: COc1cccc2cc(oc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1C[C@@H](Cl)C[C@H]1C(=O)NC(C)(C)C
InChI Key: InChIKey=RXWUXMUEFUGYFA-DIEJLHDWSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM4236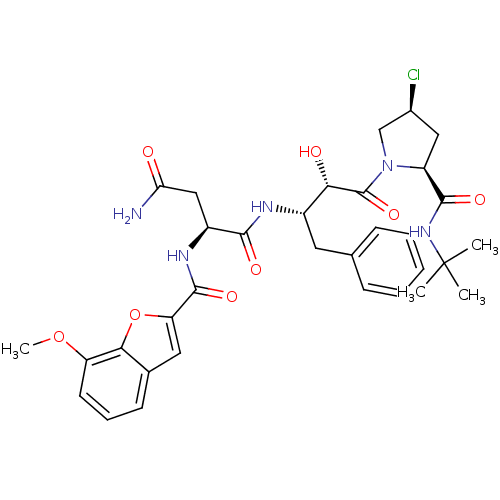 ((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | -11.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||