 Found 13 hits for monomerid = 4301
Found 13 hits for monomerid = 4301 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301
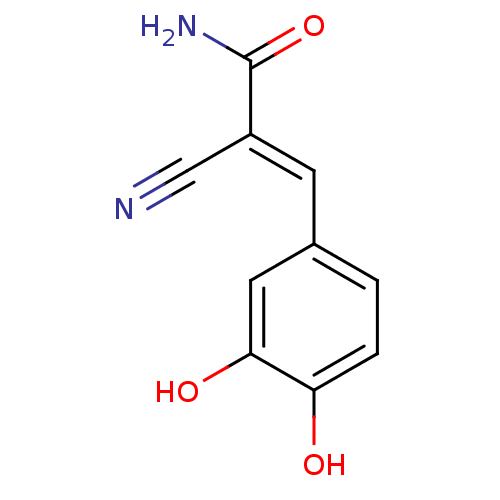
((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+3 | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem
| Assay Description
The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate. |
J Med Chem 32: 2344-52 (1989)
Article DOI: 10.1021/jm00130a020
BindingDB Entry DOI: 10.7270/Q2G44NHF |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HIV-1 integrase. |
J Med Chem 38: 4171-8 (1995)
BindingDB Entry DOI: 10.7270/Q2SB44R7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Dynamin-1
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibitory activity against dynamin1 GTPase expressed in sheep brain |
J Med Chem 48: 7781-8 (2005)
Article DOI: 10.1021/jm040208l
BindingDB Entry DOI: 10.7270/Q2BC3Z41 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against protein kinase HER-2 |
J Med Chem 46: 4657-68 (2003)
Article DOI: 10.1021/jm030065n
BindingDB Entry DOI: 10.7270/Q2N58NKR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor |
Bioorg Med Chem Lett 1: 165-168 (1991)
Article DOI: 10.1016/S0960-894X(01)80792-7
BindingDB Entry DOI: 10.7270/Q2WH2PWJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cells |
J Nat Prod 55: 1529-1560 (1992)
Article DOI: 10.1021/np50089a001
BindingDB Entry DOI: 10.7270/Q2J966CC |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... |
J Med Chem 55: 8671-84 (2012)
Article DOI: 10.1021/jm3008773
BindingDB Entry DOI: 10.7270/Q2MP54D1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against epidermal growth factor receptor (EGFR) |
J Med Chem 46: 4657-68 (2003)
Article DOI: 10.1021/jm030065n
BindingDB Entry DOI: 10.7270/Q2N58NKR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem
| Assay Description
The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate. |
J Med Chem 34: 1896-907 (1991)
Article DOI: 10.1021/jm00110a022
BindingDB Entry DOI: 10.7270/Q2KW5D7V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 1: 165-168 (1991)
Article DOI: 10.1016/S0960-894X(01)80792-7
BindingDB Entry DOI: 10.7270/Q2WH2PWJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data