

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Oc1ccc(\C=C(/C#N)C(=O)Nc2ccccc2)cc1O
InChI Key: InChIKey=HKHOVJYOELRGMV-XYOKQWHBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large T antigen (Simian virus 40) | BDBM4304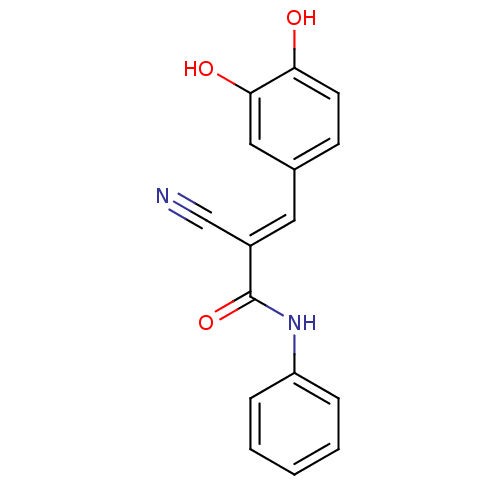 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Southern Research Specialized Biocontainment Screening Center Curated by PubChem BioAssay | Assay Description Southern Research's Specialized Biocontainment Screening Center (SRSBSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Librarie... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2ZK5F47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Hebrew University of Jerusalem | Assay Description The assay was using Swiss 3T3 cell membranes harboring EGF-R or PDGFR-beta. Receptor autophosphorylation was initiated by addition of [gamma-32P]ATP ... | J Med Chem 39: 2170-7 (1996) Article DOI: 10.1021/jm950727b BindingDB Entry DOI: 10.7270/Q2QF8R2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem | Assay Description The assay was using Swiss 3T3 cell membranes harboring EGF-R or PDGFR-beta. Receptor autophosphorylation was initiated by addition of [gamma-32P]ATP ... | J Med Chem 39: 2170-7 (1996) Article DOI: 10.1021/jm950727b BindingDB Entry DOI: 10.7270/Q2QF8R2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 6.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2JM286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 26S proteasome non-ATPase regulatory subunit 14 (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2PC30Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Inhibitory activity tested against protein kinase HER-2 | J Med Chem 46: 4657-68 (2003) Article DOI: 10.1021/jm030065n BindingDB Entry DOI: 10.7270/Q2N58NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2C (RAT-Rattus norvegicus (Rat)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2C. | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2B. | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2A. | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EGFR in human A431 cells | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center Curated by ChEMBL | Assay Description Inhibitory activity against epidermal growth factor receptor (EGFR) | J Med Chem 46: 4657-68 (2003) Article DOI: 10.1021/jm030065n BindingDB Entry DOI: 10.7270/Q2N58NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem | Assay Description The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate. | J Med Chem 34: 1896-907 (1991) Article DOI: 10.1021/jm00110a022 BindingDB Entry DOI: 10.7270/Q2KW5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM4304 ((2E)-2-cyano-3-(3,4-dihydroxyphenyl)-N-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon Curated by ChEMBL | Assay Description Inhibition of ERBB2 receptor autophosphorylation | J Med Chem 42: 3412-20 (1999) Article DOI: 10.1021/jm990199u BindingDB Entry DOI: 10.7270/Q2RR1XFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||