 Found 15 hits for monomerid = 4313
Found 15 hits for monomerid = 4313 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313
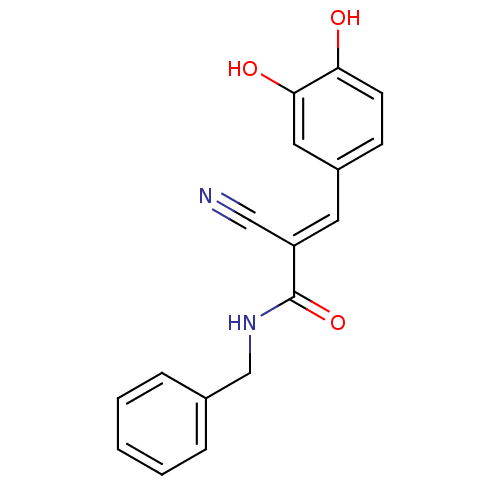
((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem
| Assay Description
The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate. |
J Med Chem 34: 1896-907 (1991)
Article DOI: 10.1021/jm00110a022
BindingDB Entry DOI: 10.7270/Q2KW5D7V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against epidermal growth factor receptor (EGFR) |
J Med Chem 46: 4657-68 (2003)
Article DOI: 10.1021/jm030065n
BindingDB Entry DOI: 10.7270/Q2N58NKR |
More data for this
Ligand-Target Pair | |
Glutamate [NMDA] receptor subunit epsilon 3/zeta 1
(RAT-Rattus norvegicus (Rat)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2C. |
J Med Chem 42: 3412-20 (1999)
Article DOI: 10.1021/jm990199u
BindingDB Entry DOI: 10.7270/Q2RR1XFD |
More data for this
Ligand-Target Pair | |
Glutamate [NMDA] receptor subunit epsilon 1/zeta 1
(Rattus norvegicus (Rat)-RAT) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2A. |
J Med Chem 42: 3412-20 (1999)
Article DOI: 10.1021/jm990199u
BindingDB Entry DOI: 10.7270/Q2RR1XFD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Inhibition of ERBB2 receptor autophosphorylation |
J Med Chem 42: 3412-20 (1999)
Article DOI: 10.1021/jm990199u
BindingDB Entry DOI: 10.7270/Q2RR1XFD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation |
J Med Chem 42: 3412-20 (1999)
Article DOI: 10.1021/jm990199u
BindingDB Entry DOI: 10.7270/Q2RR1XFD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cells |
J Nat Prod 55: 1529-1560 (1992)
Article DOI: 10.1021/np50089a001
BindingDB Entry DOI: 10.7270/Q2J966CC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uM |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1 (TDP1)
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'... |
J Med Chem 55: 8671-84 (2012)
Article DOI: 10.1021/jm3008773
BindingDB Entry DOI: 10.7270/Q2MP54D1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro inhibition of Janus kinase 3. |
Bioorg Med Chem Lett 10: 575-9 (2000)
BindingDB Entry DOI: 10.7270/Q24T6KNZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of EGFR autophosphorylation |
Proc Natl Acad Sci USA 104: 20523-8 (2007)
Checked by Author
Article DOI: 10.1073/pnas.0708800104
BindingDB Entry DOI: 10.7270/Q2DB82RH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Researchand Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Science 302: 875-878 (2003)
Article DOI: 10.1126/science.1087061
BindingDB Entry DOI: 10.7270/Q22Z16FK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Researchand Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 |
Science 302: 875-878 (2003)
Article DOI: 10.1126/science.1087061
BindingDB Entry DOI: 10.7270/Q22Z16FK |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against protein kinase HER-2 |
J Med Chem 46: 4657-68 (2003)
Article DOI: 10.1021/jm030065n
BindingDB Entry DOI: 10.7270/Q2N58NKR |
More data for this
Ligand-Target Pair | |
Glutamate [NMDA] receptor subunit epsilon 2/zeta 1
(Rattus norvegicus (Rat)-RAT) | BDBM4313

((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oregon
Curated by ChEMBL
| Assay Description
Antagonistic activity against N-methyl-D-aspartate glutamate receptor 1/2B. |
J Med Chem 42: 3412-20 (1999)
Article DOI: 10.1021/jm990199u
BindingDB Entry DOI: 10.7270/Q2RR1XFD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data