

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM43836 Glucosamine derivative, 9::MLS000580208::N-(2,5-dimethoxyphenyl)-2-(8-quinolinylsulfonylamino)benzamide::N-(2,5-dimethoxyphenyl)-2-(8-quinolylsulfonylamino)benzamide::N-(2,5-dimethoxyphenyl)-2-(quinolin-8-ylsulfonylamino)benzamide::N-(2,5-dimethoxyphenyl)-2-[(8-quinolinylsulfonyl)amino]benzamide::SMR000199740::cid_1295005
SMILES: COc1ccc(OC)c(NC(=O)c2ccccc2NS(=O)(=O)c2cccc3cccnc23)c1
InChI Key: InChIKey=QQHKDSALXVZYJY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphotransferase (Trypanosoma brucei) | BDBM43836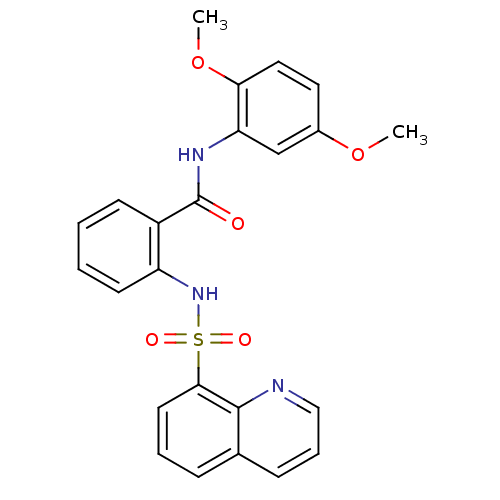 (Glucosamine derivative, 9 | MLS000580208 | N-(2,5-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -5.45 | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor-binding protein (Homo sapiens (Human)) | BDBM43836 (Glucosamine derivative, 9 | MLS000580208 | N-(2,5-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >5.30E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2VX0F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM43836 (Glucosamine derivative, 9 | MLS000580208 | N-(2,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2BV7F1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||