

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM48833 (5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-nitro-benzylidene)thiazolidine-2,4-quinone::(5Z)-3-(3-chlorophenyl)-5-[(3-methoxy-5-nitro-4-oxidanyl-phenyl)methylidene]-1,3-thiazolidine-2,4-dione::(5Z)-3-(3-chlorophenyl)-5-[(4-hydroxy-3-methoxy-5-nitrophenyl)methylidene]-1,3-thiazolidine-2,4-dione::(5Z)-3-(3-chlorophenyl)-5-[(4-hydroxy-3-methoxy-5-nitrophenyl)methylidene]thiazolidine-2,4-dione::3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-nitrobenzylidene)-1,3-thiazolidine-2,4-dione::MLS000676604::SMR000298496::cid_1231596
SMILES: COc1cc(\C=C2/SC(=O)N(C2=O)c2cccc(Cl)c2)cc(c1O)[N+]([O-])=O
InChI Key: InChIKey=PADTZIJWSMJYJO-AUWJEWJLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eukaryotic translation initiation factor 4 gamma, 1 isoform 4 (Homo sapiens (Human)) | BDBM48833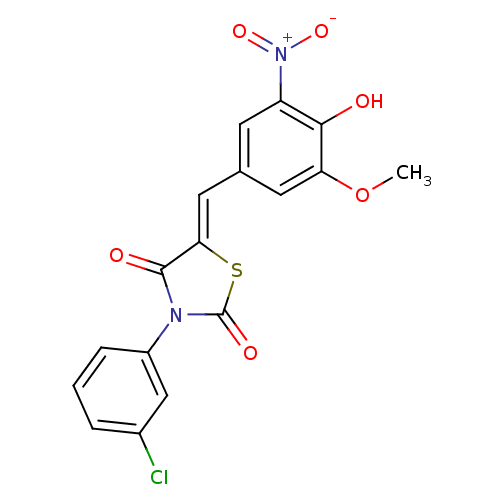 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation NIH Molecular Libraries Screening Centers Network [MLSC... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2RN3686 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BZLF2 (Human herpesvirus 4 type 2) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Theodore Jardetzky; Northw... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2057DCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q29G5K79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| poly(A) binding protein, cytoplasmic 1 (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2ZC819K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2445JXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated calcium channel subunit alpha Cav2.2 (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute Network... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2PZ578V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 9.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25M647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford- Sanford-Burnham Medical Research Institute(SBMRI, San... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2MK6BBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2CJ8BXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2SN07DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2SB447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q29C6VXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4H (Homo sapiens (Human)) | BDBM48833 ((5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q26W98HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||