

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50001579 CHEMBL3237714
SMILES: C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nn3)N3CCN(CC3)C(=O)CO)nc12
InChI Key: InChIKey=ICVHMFOFVOYFPB-IYARVYRRSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-Dependent Kinase 1 (CDK1) (Homo sapiens (Human)) | BDBM50001579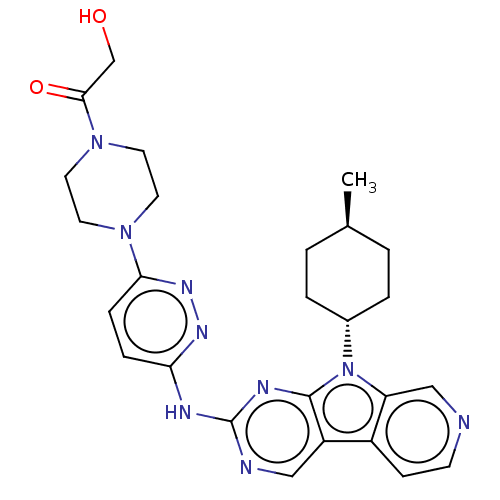 (CHEMBL3237714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of CDK1/cyclin B (unknown origin) using histone H1 as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP | J Med Chem 57: 3430-49 (2014) Article DOI: 10.1021/jm500118j BindingDB Entry DOI: 10.7270/Q2BK1DV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 4 (CDK4) (Homo sapiens (Human)) | BDBM50001579 (CHEMBL3237714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP | J Med Chem 57: 3430-49 (2014) Article DOI: 10.1021/jm500118j BindingDB Entry DOI: 10.7270/Q2BK1DV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50001579 (CHEMBL3237714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay | J Med Chem 57: 3430-49 (2014) Article DOI: 10.1021/jm500118j BindingDB Entry DOI: 10.7270/Q2BK1DV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||