

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50003127 CHEMBL122193::CHEMBL1626562::CHEMBL16659::choline salt of 5-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-3H-[1,3,4]thiadiazole-2-thione
SMILES: CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nnc(S)s1
InChI Key: InChIKey=MYNMGQGXYYHMEX-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50003127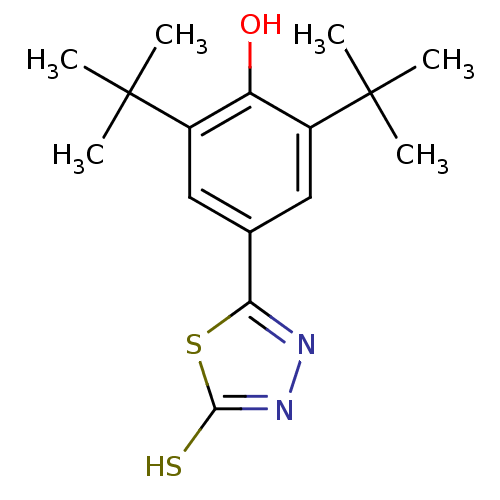 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against ovine Prostaglandin G/H synthase 1 | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 1 of human platelet rich plasma | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-2 (COX-2) (Mus musculus (Mouse)) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (RAT) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of Prostaglandin G/H synthase in intact basophilic rat leukemia cells | Bioorg Med Chem Lett 3: 2827-2830 (1993) Article DOI: 10.1016/S0960-894X(01)80773-3 BindingDB Entry DOI: 10.7270/Q2668D4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of 5-lipoxygenase in intactbasophilic rat leukemia cells | Bioorg Med Chem Lett 3: 2827-2830 (1993) Article DOI: 10.1016/S0960-894X(01)80773-3 BindingDB Entry DOI: 10.7270/Q2668D4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase measured by the reduction of leukotriene B4 (LTB4) in intact basophilic rat leukemia cells | J Med Chem 36: 1090-9 (1993) BindingDB Entry DOI: 10.7270/Q20P10PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against 5-Lipoxygenase | J Med Chem 35: 3691-8 (1992) BindingDB Entry DOI: 10.7270/Q2M61KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (RAT) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Evaluated in vitro for its inhibitory activity against Prostaglandin G/H synthase | J Med Chem 35: 3691-8 (1992) BindingDB Entry DOI: 10.7270/Q2M61KWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50003127 (CHEMBL122193 | CHEMBL1626562 | CHEMBL16659 | choli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi Curated by ChEMBL | Assay Description Inhibition of 5-LO (unknown origin) | Eur J Med Chem 92: 156-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.035 BindingDB Entry DOI: 10.7270/Q23B61TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||